Battery degradation is related to internal cracks
Scientists at the Georgia Institute of Technology used x-ray imaging techniques to observe cracks in solid-state lithium batteries

Scientists at the Georgia Institute of Technology used x-ray imaging techniques to observe cracks in solid-state lithium batteries. They say the findings have changed people's understanding of solid-state battery performance and may lead to more durable systems.
Solid-state batteries have the potential to provide safer, smaller alternatives to today's lithium-ion technology, and research teams around the world are working to help commercialize the technology.
Scientists at the Georgia Institute of Technology have found a finding that will help push such research in the right direction. The team built a solid-state battery with a solid ceramic layer between the two layers of lithium as an electrolyte, and then they used x-ray computed tomography, a medically similar CT scan technique to observe charge and discharge. Reaction and degradation in the process.
The researchers said, "It's not easy to figure out how to combine these solid parts together and perform well over time." "We are studying how to design the interfaces between these solid parts and let them be used for as long as possible." ""
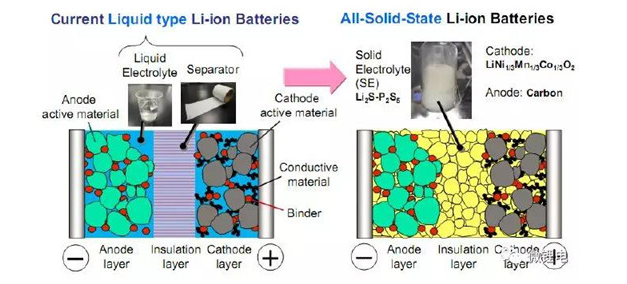
Liquid lithium ion battery (left) solid state lithium ion battery (right)
The results of the study were published in the journal "Visualizing Chemomechanical Degradation of a Solid-State Battery Electrolyte" in the Journal of ACS Energy Letters. How does the layer begin to form cracks within a few days resulting in increased resistance to ion currents.
The researchers said that it was previously believed that the chemical reaction between the lithium metal and the electrolyte interface was responsible for the degradation of the battery, rather than cracks inside the battery. But they learned through imaging that in this particular material, not the chemical reaction itself has problems, they do not affect the performance of the battery, but the breakage of the cell destroys the performance of the cell.
Researchers say their findings may also apply to the chemical composition of solid-state batteries. In ordinary lithium-ion batteries, the materials we use determine how much energy we can store. Pure lithium has the largest capacity, but its compatibility with liquid electrolytes is not good. If solid lithium and solid electrolytes can be used, that would be the ultimate in energy density.
